Link Your Antibody
to its Fullest
Potential
services to include bioconjugation and the development and manufacturing
of antibody-drug conjugates (ADCs).
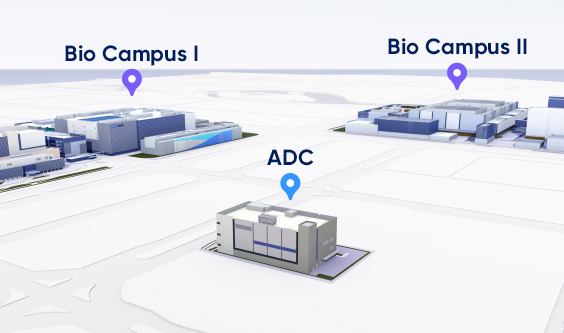
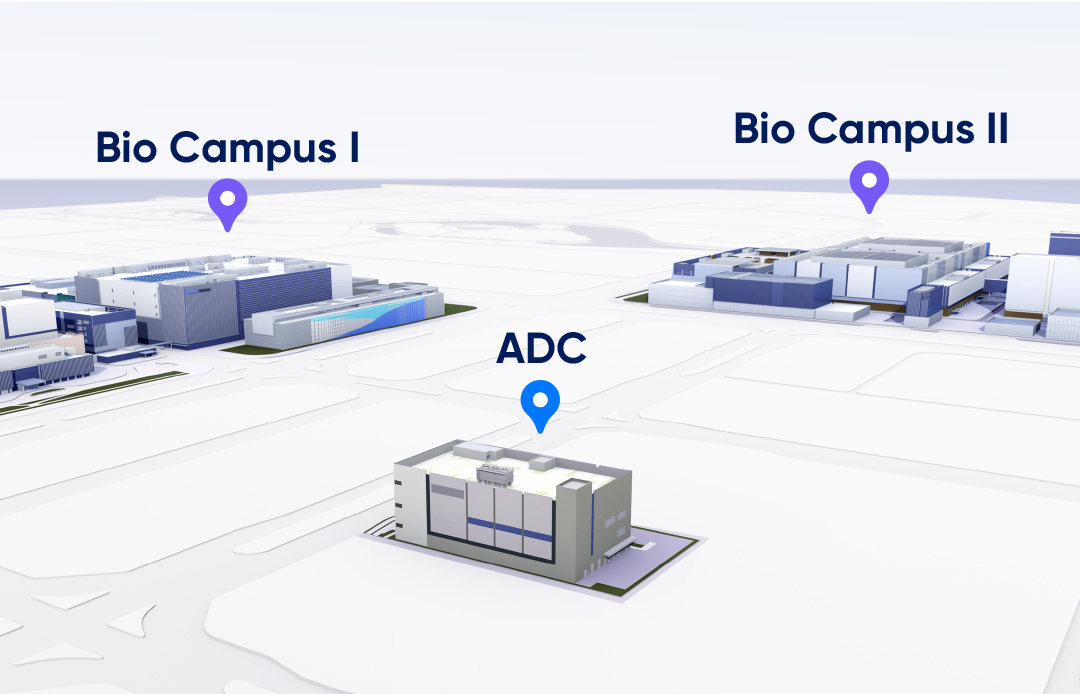
Expected to be operational in December 2024, our standalone bioconjugation
facility will be supported by the robust GMP systems established for our
existing high-capacity clinical and commercial biological manufacturing. It is
located directly across from our main manufacturing sites.
Dedicated ADC Facility
-
- Expedited drug substance (DS) delivery with flexibility at a single site
- Expertise in quality and proven operational track record
- Integrated systems with established tools and procedures
- Stable supply chain management system
- Features
-
- Single-use and stainless steel reactors
(up to 500 L conjugation train) - Full-scope laboratories for ADC development,
quality control (QC), and MSAT - High containment facilities for high-potency APIs (HPAPIs)
- Occupational Exposure Limit (OEL) target of 5ng/m³
- Single-use and stainless steel reactors
Dependable ADC Services From a Trusted CDMO
We offer comprehensive services that range from antibody production and conjugation development to manufacturing.
Agile ADC Development Services
Our agile development process reflects the need for speed and efficiency in the early stages of ADC development. With specialized teams, we help our clients move from cell line to formulation in as little as 15 months.
-
Late discovery
Tool box options for the best development choice
-
Conjugation
Method, parameter optimization, DAR variation control
-
Analysis
ADC Method development, Precise Analysis and Characterization
-
Formulation
Stability enhancement and Lyo development
Concurrent development of mAb and ADC
- Cell Line Development
- mAb Process Development
- ADC Process Development
- Conjugation parameter development
- Downstream process development
- ADC Formulation development
- Analytical method development
- Non GMP mAb Tox, Non GMP ADC DS
- GMP mAb DS, GMP ADC DS
- 15 months
Flexible ADC Manufacturing Processes
Samsung Biologics’ new state-of-the-art ADC facility is designed to support all aspects of ADC manufacturing. Our team constructs timelines that accommodate all ADC components, customizing the journey for each client, from mAb thawing through to formulation and bulk fill-finish.
Payload & mAb Prep
- mAb Thawing
- Payload Prep
- UF/DF1
- Reduction
Conjugation
- Conjugation
- Quenching
- UF/DF2
- Chroma- tography
Formulated Bulk
- UF/DF3
- Formulation & Bulk Fill
Integrated ADC Analytical Testing
With Samsung Biologics, all of the analytical tests required for ADC development can be performed at a single site in close partnership with the ADC development and manufacturing teams. This simplicity streamlines your experience and ensures rigorous quality throughout the process.
-
Raw Material
- ID for payload/linker
-
IPC Testing
- Protein concentration
-
Chemistry
- DAR
- Residual solvent
- Free drug
-
Biochemistry
- Bioassay
- Antigen binding assay
- Cytotoxidity assay
Samsung Life Science Fund and securing future partnerships.
conjugation technology
development technology
technology
technology for ADC
